1. How do you define anaphylaxis? When and how do you give a patient with anaphylaxis epinephrine? How does age or past medical history factor in your decision?
 Anaphylaxis is an allergic reaction, with rapid onset after contact with the offending allergen, which may cause death. In short, the criteria for this clinical diagnosis are acute onset of skin/mucosal involvement (seen in 80-90% of patients, not all) with respiratory compromise and/or reduced blood pressure (Sampson, 2006).
Anaphylaxis is an allergic reaction, with rapid onset after contact with the offending allergen, which may cause death. In short, the criteria for this clinical diagnosis are acute onset of skin/mucosal involvement (seen in 80-90% of patients, not all) with respiratory compromise and/or reduced blood pressure (Sampson, 2006).
There are basically no modern, placebo-controlled, randomized trials for any therapies we use for humans experiencing anaphylaxis. This is in part because of the life-threatening nature of the disease, the sense from case series and clinical practice that epinephrine works, and the difficulty of obtaining consent from a hypotensive patient in respiratory distress who is also vomiting (Sheikh, 2009).
Patients with anaphylaxis should get epinephrine now, not later. Cases series of 13, and 124, respectively, fatal and near fatal anaphylactic reactions (Sampson, 1992; Bock, 2001) showed that patients who received epinephrine within thirty minutes of symptoms were more likely to live, and that of patients who died, very few received any epinephrine during their treatment.
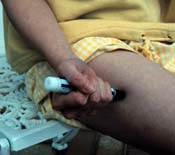
The method of administering epi is clear: intramuscular injection into the antero-lateral thigh. Studies in children and adults not experiencing anaphylaxis (n.b.) have shown a more rapid increase in plasma and tissue epi levels with this mode, even over IM deltoid injection (Simons 2001; Simons, 1998).
There are NO absolute contraindications to the use of epinephrine in anaphylaxis. Many practitioners are concerned about side effects of administering epi in the elderly, especially those with CAD or cardiac risk factors. Remember, however, that the heart is a target in anaphylaxis itself, which can worsen CAD, can cause MI, or cause dysrhythmia. Most experts feel this cardiac risk from untreated anaphylaxis, coupled with the risk or death or other serious illness from anaphylaxis, mean that epinephrine’s benefits outweigh this theoretical risk, and give it in such a situation (Sheikh, 2009).
2. When do you consult ENT to scope a patient with an allergic reaction/anaphylaxis?
The literature does not really address this question. Our practice is that ENT should be called early when there is any question of airway involvement, obviously with stridor, uvular or other apparent airway edema, voice changes, and so on. However, the time course of anaphylaxis is such that by the time a consultant arrives with their scope the situation may have progressed to where you are sweating and looking at the cric kit. All of this is to say: call ENT early, as their visualization of the cords when airway involvement is equivocal is of utility, as are serial viewings to see edema resolving. In the scenario of progression, especially after IM epi, however, please proceed to question three.
3. How do you make the decision to intubate a patient with anaphylaxis?
In a word, early. If you’ve never seen severe and progressive anaphylaxis, please believe us when we say you should always have a difficult airway box at the bedside and ready whenever anyone with even a hint of respiratory involvement comes to you. Over seconds to minutes, patients can go from phonating with slight voice change to complete occlusion of the upper airway with severe bronchospasm. Be ready to cric or perform a trach, emergently. Also, in cases such as these, when patients are not responsive to IM epi, an epinephrine drip is indicated. Although this is slightly beyond the purview of this EM Lyceum topic, we like this video for how to make your own epi drip (in a pinch).
4. Which patients do you admit? How long do you observe the patients you intend to discharge?
Any patient with refractory anaphylaxis or who required respiratory intervention should be admitted, without passing go, to the ICU. For the majority of patients, though, the disposition question is one of observation time. Other factors in determining disposition are the feasibility of getting an Epi-pen at the time of discharge (e.g., pharmacy access in the middle of the night), ease of returning to the ED if there is a recurrence of symptoms, and history of biphasic anaphylaxis.
Biphasic reactions are a common concern, wherein the symptoms recur after the initial medications have worn off, or due to a second wave of immune response to an allergen. This phenomenon has been described from 1 hour to 3 days after initial exposure to the allergen (Douglas, 1994), a fact that must be communicated to patients discharged to home. From case series, it is theorized that biphasic anaphylaxis occurs in 1-20% of all cases, and that it is closer to 20% in the more severe cases, especially those requiring higher doses of epi. The expert consensus on anaphylaxis, and the practice of most physicians in our departments, is to observe patients who are symptom free for a period of 4-6 hours in the department (but of course warn them, on discharge, of the possibility of a delayed biphasic recurrence) (Sampson, 2006; Tole, 2007; Kemp, 2008).